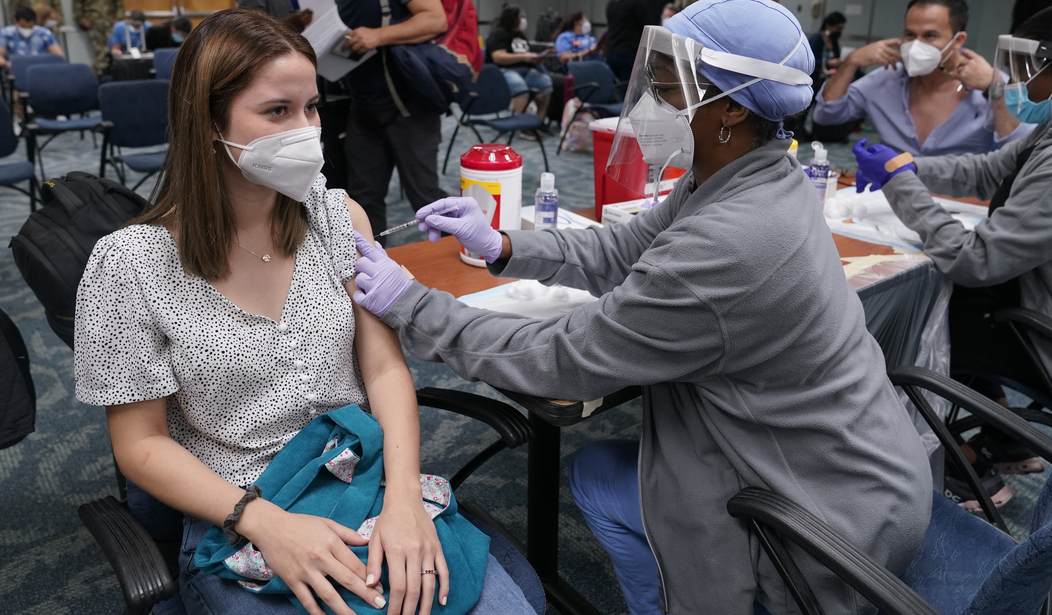
Pfizer’s “sprint” to the finish line for approval from the Food and Drug Administration that started in May is finally over and the company has been granted full approval for their COVID-19 vaccine, allowing them to give doses to children as young as 16.
According to CBS News, all the “regulatory hurdles,” including safety inspection of the vaccine factories around the world and the company’s data on the vaccine, have been completed. With FDA approval, health officials hope that the slowdown of vaccinations will pick back up as confidence in the vaccine may rise with this stamp of approval.
Other companies are not too far behind Pfizer according to CBS News. Moderna and Johnson and Johnson are also going through the motions in their approval processes.
The FDA’s been under intense pressure to speed through with the approval process as cases rose among the unvaccinated thanks to the Delta variant. Some companies and schools have been waiting for approval from the FDA before they began mandating those under their umbrella be vaccinated. This includes companies like United Airlines and the University of Minnesota.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.” said the FDA’s Acting Commissioner Janet Woodcock in a statement.
“I actually think it’s going to end up making a pretty big difference,” said Dr. Ashish Jha of Brown University School of Public Health. “For some people, the emergency use has been a barrier to getting vaccinated, and a lot of companies and businesses and schools, they have been waiting for full approval before mandating vaccines.”
According to the CDC, 92 million Americans have already taken the Pfizer doses. Roughly half of America is fully vaccinated from one company or another.
While it’s likely vaccination rates will rise to some degree, many have refused the jab for legitimate reasons. Be it that they’ve already had the virus recently, have medical concerns regarding the side effects of the vaccine, or the fact that there hasn’t been enough time to see its long-term effects, many will still continue to refuse to get the shot.
FDA approval is great, but there’s no substitute for time. The fact that the approval process was a rush job isn’t going to make people feel any better. Regardless, you can expect the FDA approval to put many at ease and give companies and schools to push the vaccine on their associates and employees.













Join the conversation as a VIP Member